|
|
|
|
• Uniform • Galvanic • Crevice • Pitting • Intergranular • SSC • LME • MIC • SCC • HB-HE-HIC-HMx • Fatigue • Erosion • Stray Current • Index |
|
Different Types of
Corrosion
|
|
Pitting Corrosion |
|
|
Recognition of Pitting Corrosion |
|
|
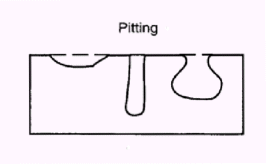
What is pitting corrosion? Pitting Corrosion is the localized corrosion of a metal surface confined to a point or small area, that takes the form of cavities. Pitting corrosion is one of the most damaging forms of corrosion. Pitting factor is the ratio of the depth of the deepest pit resulting from corrosion divided by the average penetration as calculated from weight loss. The following photo shows pitting corrosion of a SAF2304 duplex stainless steel after exposure to 3.5% NaCl solution.
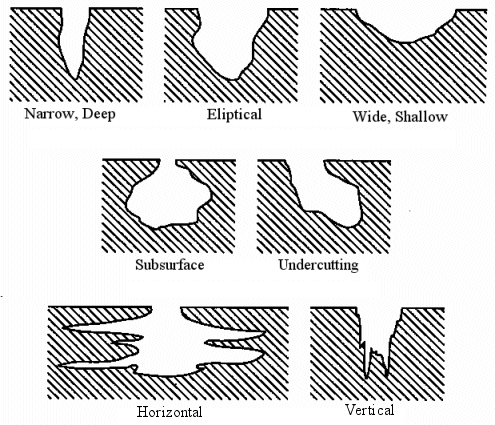
What materials are susceptible to pitting corrosion? Pitting corrosion is usually found on passive metals and alloys such aluminium alloys, stainless steels and stainless alloys when the ultra-thin passive film (oxide film) is chemically or mechanically damaged and does not immediately re-passivate. The resulting pits can become wide and shallow or narrow and deep which can rapidly perforate the wall thickness of a metal.
ASTM-G46 has a standard visual chart for rating of pitting corrosion.
The shape of pitting corrosion can only be identified through metallography where a pitted sample is cross-sectioned and the pit shape, the pit size, and the pit depth of penetration can be determined.
|
|
|
Mechanisms of Pitting
Corrosion |
|
|
What causes pitting corrosion? For a defect-free "perfect" material, pitting corrosion is caused by the ENVIRONMENT (chemistry) that may contain aggressive chemical species such as chloride. Chloride is particularly damaging to the passive film (oxide) so pitting can initiate at oxide breaks.
The environment may also set up a differential aeration cell (a water droplet on the surface of a steel, for example) and pitting can initiate at the anodic site (centre of the water droplet).
For a homogeneous environment, pitting IS caused by the MATERIAL that may contain inclusions (MnS is the major culprit for the initiation of pitting in steels) or defects. In most cases, both the environment and the material contribute to pit initiation.
What are the factors
influencing pitting corrosion?
The ENVIRONMENT (chemistry) and the MATERIAL
(metallurgy) factors determine whether an existing pit can be repassivated
or not. Sufficient aeration (supply of oxygen to the reaction site) may
enhance the formation of oxide at the
pitting site and thus repassivate or
heal the damaged passive film (oxide) - the pit is repassivated and no
pitting occurs. An existing pit can also be repassivated if the material
contains sufficient amount of alloying elements such as Cr, Mo, Ti, W, N,
etc.. These elements, particularly Mo, can significantly enhance the
enrichment of Cr in the oxide and thus heals or repassivates the pit. More
details on the alloying effects can be found in the technical paper on
"Stainless Steels and Alloys: Why They Resist
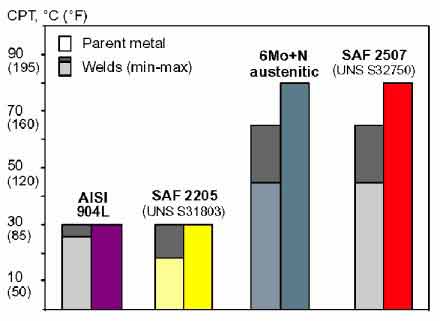
How to evaluate the resistance
of an alloy to pitting corrosion?
A material's resistance to pitting corrosion is usually evaluated and ranked
using the critical
pitting temperature (CPT)
in accordance with the ASTM Standard
|
|
|
Prevention of Pitting Corrosion |
|
|
How to prevent pitting corrosion? Pitting corrosion can be prevented through:
|
|
|
Modeling and Prediction of
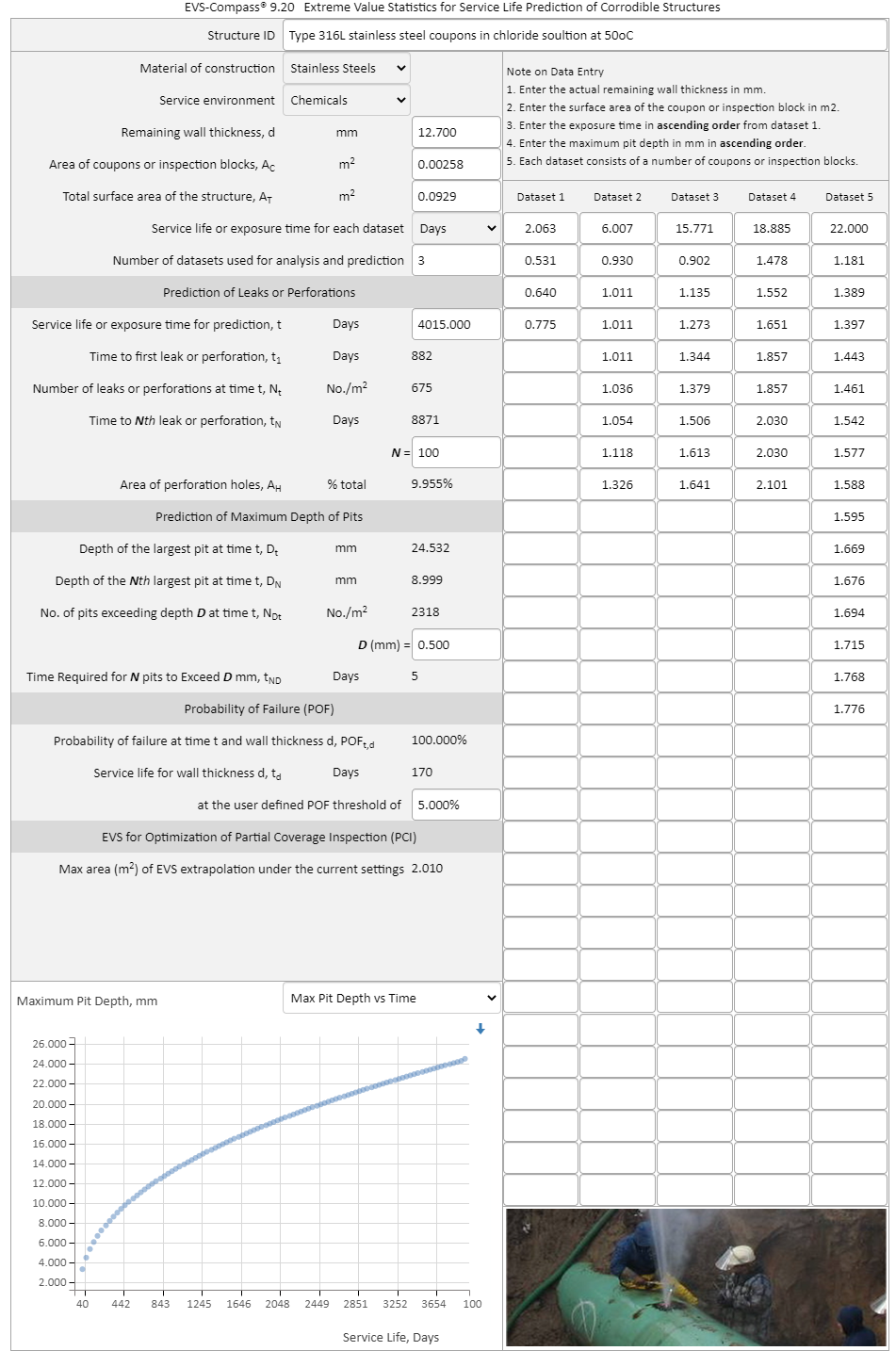
Pitting Corrosion EVS-Compass®: Extreme Value Statistics for Corrosion Modeling and Corrosion Life Prediction Extreme value statistics (EVS) has been used since the 1950s for extrapolating corrosion damages (maximum pit depth, crevice depth, crack depth etc.) from small lab samples, field coupons, or partial coverage inspection blocks to larger area of structures and assets at present or future times. WebCorr's EVS-Compass is the only device and OS independent EVS software on the market for corrosion modeling and life prediction of corrodible structures. Designers, OEM engineers, consultants, operation personnel, maintenance and inspection engineers, and government regulators can quickly and accurately determine:
CRA-Compass®: Your Guide to
Corrosion Resistant Alloys Overview of CRA-Compass for Waters and Brines This module deals with the application limits of 55 common corrosion resistant alloys used in water systems including natural seawater, chlorinated seawater, brines, produced water, formation water, brackish water, groundwater, fresh water, and potable water. Users can define their own alloys for CRA-Compass to evaluate the application limits for their resistance to pitting, crevice corrosion, and stress corrosion cracking (SCC) under the specified operating conditions. The performance of the CRAs in coastal/marine environment is also included in this module. More detailed information on CRA-Compass is available here. CIPAL-Compass: Copper-Induced Pitting in Aluminium Alloys This software predicts pitting depth, pitting rate and time to perforation of aluminum alloys in contact waters and process fluids that contain trace amount of copper ions.
|
|
|
For more details on Pitting
Corrosion |
|
Where can I learn more about
pitting corrosion? More details on pitting corrosion are included in the following
corrosion courses which you can take as
in-house training courses,
course-on-demand, online
courses or distance
learning courses:
If you require corrosion expert witness or corrosion consulting service on pitting corrosion, our NACE certified Corrosion Specialist is able to help. Contact us for a quote. |
|
| Home | Subject Index | Contact Us | PDF |
Copyright © 1995-2025.. All rights reserved. |