|
|
|
|
• EC • Season Cracking • Caustic Cracking • Intergranular • SSC • LME • MIC • SCC • HB-HE-HIC-HMx-HTHA • Fatigue • Stray Current •Index |
|
Different Types of
Corrosion
|
|
Hydrogen Embrittlement (HE) |
|
|
Recognition of Hydrogen Embrittlement |
|
|
What is hydrogen embrittlement? Hydrogen embrittlement (HE) is a process resulting in a decrease of the toughness or ductility of a metal due to the presence of atomic hydrogen. Hydrogen embrittlement has been recognized classically as being of two types.
The first, known as internal hydrogen embrittlement, occurs when the hydrogen enters molten metal which becomes supersaturated with hydrogen immediately after solidification.
The second type, environmental hydrogen embrittlement, results from hydrogen being absorbed by solid metals. This can occur during elevated-temperature thermal treatments and in service during electroplating, contact with maintenance chemicals, corrosion reactions, cathodic protection, and operating in high-pressure hydrogen.
|
|
|
Mechanisms of Hydrogen Embrittlement |
|
|
What causes hydrogen embrittlement? In the absence of residual stress or external loading, environmental hydrogen embrittlement is manifested in various forms, such as blistering, internal cracking, hydride formation, and reduced ductility. With a tensile stress or stress-intensity factor exceeding a specific threshold, the atomic hydrogen interacts with the metal to induce subcritical crack growth leading to fracture. In the absence of a corrosion reaction (polarized cathodically), the usual term used is hydrogen-assisted cracking (HAC) or hydrogen stress cracking (HSC).
In the presence of active corrosion, usually as pits or crevices (polarized anodically), the cracking is generally called stress-corrosion cracking (SCC), but should more properly be called hydrogen-assisted stress-corrosion cracking (HSCC). Thus, HSC and electrochemically anodic SCC can operate separately or in combination (HSCC). In some metals, such as highs-strength steels, the mechanism is believed to be all, or nearly all, HSC. The participating mechanism of HSC is not always recognized and may be evaluated under the generic heading of SCC.
|
|
|
Modeling, Prediction and Prevention of Hydrogen Embrittlement |
|
|
How to select alloy steels for resistance to low temperature hydrogen
damages?
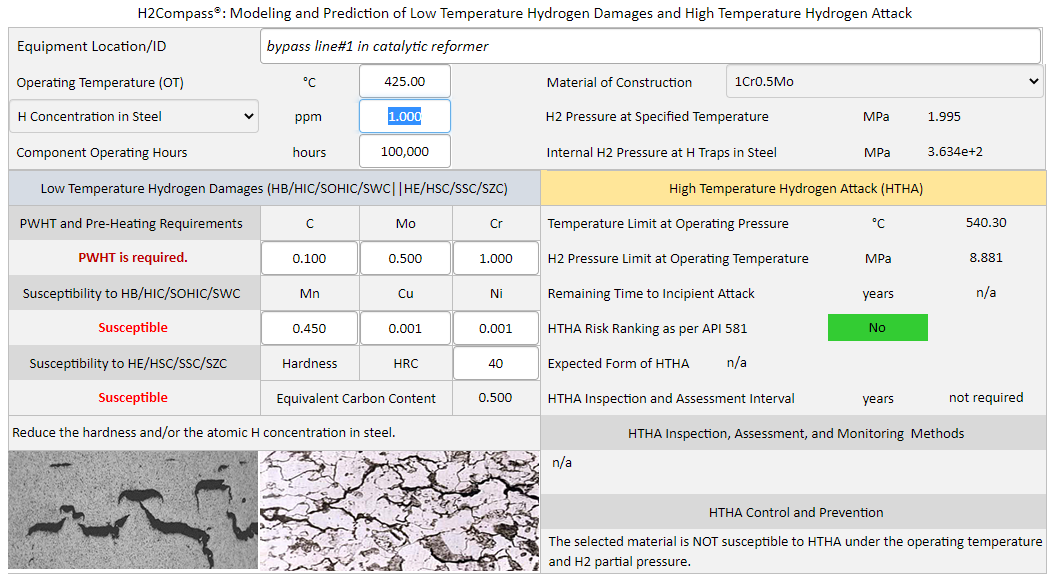
H2Compass is a powerful software for modeling and prediction of low temperature hydrogen damages and high temperature hydrogen attack (HTHA). H2Compass software provides instant answers to the above questions. The software can be used to determine the atomic hydrogen concentration in steels, the internal hydrogen gas pressure at hydrogen traps in steels, the steel's susceptibility to low temperature hydrogen damages, and the requirements for post-weld heat treatment (PWHT) and pre-heating.
How to prevent hydrogen embrittlement? Hydrogen embrittlement can be prevented through:
|
|
|
For more details on Hydrogen Embrittlement |
|
|
Where can I learn more about hydrogen embrittlement? More details on environmental cracking are included in the following corrosion courses which you can take as in-house training courses, course-on-demand, online courses or distance learning courses:
If you require corrosion expert witness or corrosion consulting service on hydrogen embrittlement, our NACE certified Corrosion Specialist is able to help. Contact us for a quote. |
|
|
Home | Subject Index | Contact Us | PDF |
Copyright © 1995-2025.. All rights reserved. |