|
|
|
|
• Uniform • Galvanic • Crevice • Filiform • Pitting • EC • Intergranular • SSC • LME • MIC • SCC • HB-HE-HIC-HMx • Fatigue • Erosion • Index |
|
Different Types of
Corrosion
|
|
|
Crevice Corrosion |
|
|
Recognition of Crevice Corrosion |
|
|
How to identify crevice corrosion? The damage caused by crevice corrosion is normally confined to one metal at localized area within or close to the joining surfaces.
In this photo, a type 316 stainless steel tube and tube sheet from a heat exchanger in a seawater reverse osmosis (SWRO) desalination plant suffered crevice corrosion due to the presence of crevice (gap) between the tube and tube sheet.
|
|
|
Mechanisms of Crevice Corrosion |
|
|
What causes crevice corrosion? Crevice corrosion is initiated by a difference in concentration of some chemical constituents, usually oxygen, which set up an electrochemical concentration cell (differential aeration cell in the case of oxygen). Outside of the crevice (the cathode), the oxygen content and the pH are higher - but chlorides are lower.
Chlorides concentrate inside the crevice (the anode), worsening the situation. Ferrous ions form ferric chloride and attack the stainless steel rapidly. The pH and the oxygen content are lower in the crevice than in the bulk water solution, just as they are inside a pit. The pH inside the crevice may be as low as 2 in a neutral solution. Once a crevice has formed, the propagation mechanism for crevice corrosion is the same as for pitting corrosion.
The major factors influencing crevice corrosion are:
What materials are susceptible to crevice corrosion? All film-forming alloys whose corrosion resistance depend on the stability of passive films are particularly susceptible to crevice corrosion. Examples are all grades of stainless steels and aluminium alloys.
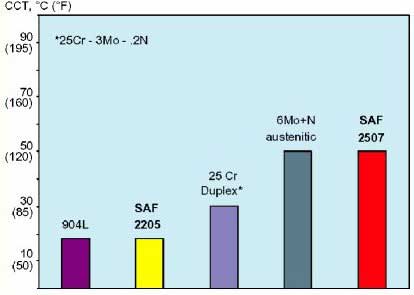
How to evaluate a material's resistance to crevice corrosion? A material's resistance to crevice corrosion is usually evaluated and ranked using the critical crevice temperature (CCT) in accordance with the ASTM Standard G48-03: Standard Test Methods for Pitting and Crevice Corrosion of Stainless Steels and Alloys by Use of FeCl3. The critical crevice temperature is the minimum temperature (°C) to produce crevice attack and CCT is usually lower than the critical pitting temperature (CPT). Computer software such as CRA-Compass can be used to assess the crevice corrosion resistance of an alloy for a given temperature and chloride concentration.
|
|
|
Prevention and Prediction of Crevice Corrosion |
|
|
How to prevent crevice corrosion? Crevice corrosion can be designed out of the system
How to predict crevice corrosion? Computer software such as CRA-Compass can be used to predict crevice corrosion for a given alloy at a given temperature and chloride concentration.
|
|
|
For more details on Crevice Corrosion |
|
|
Where can I learn more about crevice corrosion? More details on crevice corrosion are included in the following corrosion courses which you can take as in-house training courses, course-on-demand, online courses or distance learning courses:
If you require corrosion expert witness or corrosion consulting service on crevice corrosion, our NACE certified Corrosion Specialist is able to help. Contact us for a quote. |
|
|
Home | Go to Top | Contact Us | PDF |
Copyright © 1995-2025.. All rights reserved. |