|
|
|
|
EC Caustic Embrittlement Intergranular SSC LME MIC SCC HB-HE-HIC-HMx-HTHA Sulfidation NAC Erosion Fretting Stray Current Index |
|
Different Types of
Corrosion
|
|
High-Temperature Sulfidation (Sulfidic) Corrosion |
|
|
|
|
|
Sulfidation (Sulfidic) corrosion refers to the corrosion of a metal resulting from reaction with sulfur compounds in high temperature environments such that a surface sulfide scale forms often with sulfur penetrating somewhat below the original thickness.
Sulfidation is also often referred to as sulfidic corrosion in the corrosion literature. In the presence of H2/H2S, sulfidation is frequently referred to as H2-H2S corrosion.
Sulfidation corrosion can occur wherever sulfur compounds are present in a hydrocarbon stream and the temperature exceeds about230°C. H2/H2S corrosion can also occur in the absence of hydrocarbon.
|
|
|
Mechanisms of High Temperature Sulfidation, Sulfidic Corrosion, H2-H2S Corrosion |
|
|
There are two separate and distinct mechanisms
of sulfidation corrosion. One occurs where H2 is present in addition to the
sulfidation-causing sulfur species, as is common in many refining processes,
such as hydrotreating and hydrocracking. The other occurs in the absence of
H2 (hydrogen free) in processing units that do not employ H2 as a component
of the process. They both are non-aqueous, diffusion-based corrosion
mechanisms that occur at elevated temperature. There is considerable debate
in the industry as to the correct threshold temperature for hydrogen-free
Four distinct steps have been identified in the sulfidation mechanism in H2-free services: (1) Adsorption of the sulfur compounds on the scale surface. (2) Catalyzed decomposition of the sulfur compounds, and inclusion of sulfur in the FeSx scale lattice, resulting in the formation of additional cation vacancies and electron holes. (3) Diffusion of cation vacancies and electron holes to the FeSx/Fe interface. (4) Reaction at the FeSx/Fe interface. Fe oxidizes to form the scale, thus reducing the concentration of cation vacancies and electron holes:
The corrosion rate is normally limited by one of these steps. In H2-free environments, it is thought that step (1) or (2) is the rate-limiting step. Studies have shown that some sulfur compounds more readily absorb (or chemisorb) into the sulfide scale than even H2S, and thus exhibit greater corrosion rates when compared to H2S. Chromium in the steel reportedly poisons the catalytic decomposition of sulfur compounds in step (2) and thus accounts for the improvement in corrosion resistance of steel alloyed with Cr. The diffusion flux of cation vacancies and electron holes through the spinel phase (FeCr2S4) is less than through the FeSx, slowing step (3) and thus limiting corrosion rates.
Sulfidation in Hydrogen-Hydrogen Sulfide Environments: Mechanism of H2-H2S Corrosion
The composition and morphology of the iron sulfide scales formed in H2-H2S environments are essentially the same as those formed by H2-free sulfidation. However, the reason for the disparate performance of alloys such as 5% Cr in H2-free sulfidation and H2-H2S services has not been clearly established. One suggestion is that in the presence of H2, other less corrosive sulfur compounds were converted into H2S, and the higher H2S concentrations were the reason for the increased corrosion rates. However, process-plant experience and laboratory tests have given contradictory or conflicting results showing that several of these other sulfur compounds (e.g., disulfides, mercaptans) are actually more corrosive than H2S. H2 promotes the decomposition of various absorbed sulfur compounds, counteracting the influence of Cr alloying additions and thus resulting in accelerated corrosion rates (step 2 in the four-step process). This is thought to be a reason that Cr-steel alloys are no more resistant to H2-H2S corrosion than CS. The poisoning effect of Cr on the catalytic generation of H2S (step 2 in the four-step process) is another possible reason why Cr steel alloys show better resistance to sulfidation in H2-free environments while showing only slight or minimal improvement in H2-H2S corrosion.
|
|
|
Modeling, Prediction and Prevention of High Temperature Sulfidation (Sulfidic) Corrosion |
|
|
How to determine the operating H2S concentration limits for steels in sulfur-containing environments to avoid high temperature sulfidation (sulfidic) corrosion? How
to select alloy steels for high temperature sulfidation resistance?
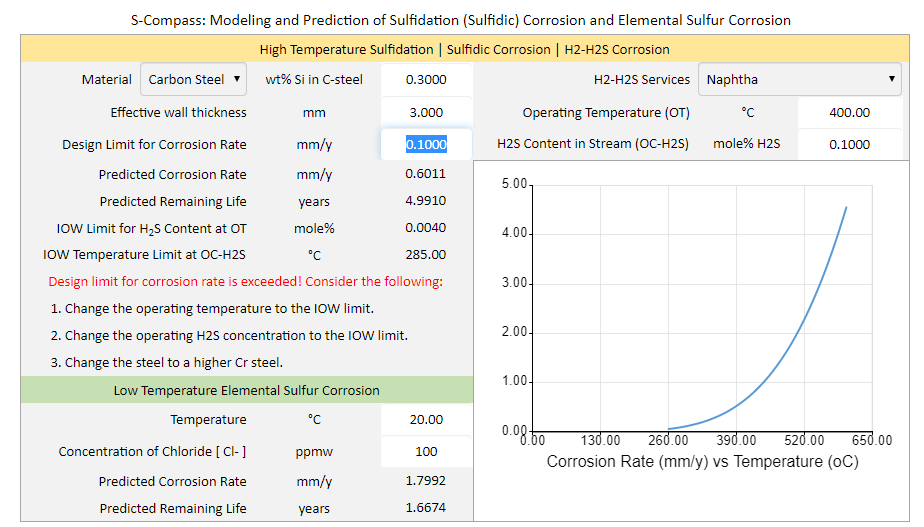
S-Compass is a powerful software for modeling and prediction of high temperature sulfidation (sulfidic) corrosion. S-Compass software provides instant answers to the above questions. The software can be used to determine the safe operating limits of temperature and hydrogen sulfide (H2S) concentration for carbon steels, low alloy steels, and stainless steels commonly used in the refining and petrochemical industries.
How to prevent High-temperature sulfidation (sulfidic) corrosion? High-temperature sulfidation (sulfidic) corrosion can be prevented through: |
|
|
For more details on High Temperature Sulfidation (Sulfidic) Corrosion |
|
|
Where can I learn more about high temperature sulfidation (sulfidic) corrosion? More details on high-temperature sulfidation (sulfidic) corrosion are included in the following corrosion courses which you can take as in-house training courses, course-on-demand, online courses or distance learning courses:
If you require corrosion expert witness or corrosion consulting service on high temperature sulfidation (sulfidic) corrosion, our NACE certified Corrosion Specialist is able to help. Contact us for a quote. |
|
| Home | Subject Index | Contact Us | PDF |
Copyright © 1995-2024. All rights reserved. |
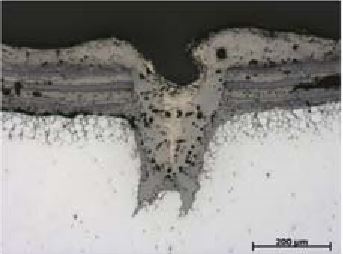 What causes
sulfidation (sulfidic) corrosion?
What causes
sulfidation (sulfidic) corrosion?