Stainless Steels and Alloys: Why They Resist Corrosion
and How They Fail*
Effect of Alloying Elements
Each grade of stainless steel has its own
unique property due to modifications to its composition or structure. The common
requirement for all grades of stainless steels is that the chromium content must
be greater than 11% (wt) in the composition. This is the minimum amount of
chromium that can maintain the "stainless" appearance of a steel by forming a
compact chromium-rich ultra thin surface oxide, know as "passive film". Another
major alloying element commonly found in austenitic and duplex stainless steels
is nickel. As a more noble element than iron, nickel in stainless steels help
improve the corrosion resistance. Fig.3 shows the polarization behavior of the
nickel-containing austenitic 304 stainless steel and two ferritic grades in
sulphuric acid [4,8,9].
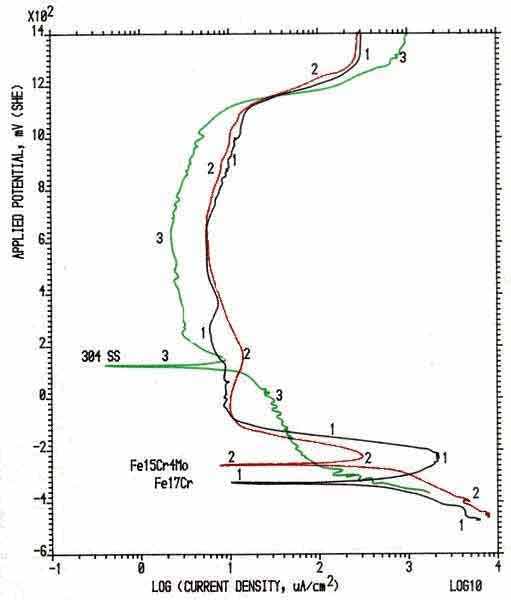
Fig.3 Polarization Behavior of Austenitic and Ferritic
Stainless Steels It is clearly seen from the above figure that the 9% nickel
in 304 stainless steel has a corrosion potential that is over 400 mV more
positive or noble than the ferritic Fe17Cr stainless steel. This shift
of corrosion potential in the noble direction indicates an increased thermodynamic
stability of the metal/solution system. Another marked feature observed
from this polarization diagram is that the peak passivation current density
for the nickel-containing 304 steel is reduced by over 2 orders of magnitude
when compared with the ferritic Fe17Cr. Nickel in the alloy is also able
to reduce the passive current density within the passive potential range.
Molybdenum addition in stainless steels increases the
resistance to localized corrosion such pitting and crevice. The polarization
curve in Fig.3 also showed that 4% molybdenum can reduce the peak passivation
current density by an order of magnitude.
|
