|
Home | Consulting | Training | Expert Witness | Failure Analysis | Design Review | Corrosion Test | Corrosion Software | Protective Coatings | Materials Selection | Cathodic Protection | >>> | |
 Corrosion Modeling Software and Corrosion Prediction Software Series GC-Compass®: A Software Tool for Galvanic Corrosion Prediction and Materials Compatibility Assessment The Ultimate Software Solution to Costly Galvanic Corrosion Version 12.4 (6,555 Galvanic Couples)
Anytime Anywhere Any Device Any OS No USB dongles No installation No Browser Plug-ins
|
|
|
Why WebCorr |
Performance Guarantee |
Unparalleled Functionality |
Unmatched Usability |
Any Device Any OS |
Free Training
& Support |
CorrCompass |
|
|
|
|
|
GC-Compass is the only device and OS independent software tool on the market for the prediction of galvanic corrosion and assessment of materials galvanic compatibility. The current version has 6,555 galvanic couples covering every possible coupling of engineering materials in use today. Designers, engineers, architects, consultants, or maintenance and inspection personnel can quickly assess and quantify the impact of galvanic coupling of dissimilar metals, alloys, and CRFP composites on the remaining life of their components or systems anytime, anywhere, on any device running any OS without the need to install or download anything.
The NACE/ASTM G193 standard defines corrosion as "the deterioration of a material, usually a metal, that results from a chemical or electrochemical reaction with its environment."
Input parameters in GC-Compass include:
GC-Compass models the effects of the above input parameters and predicts the following:
Users of GC-Compass start by selecting the dissimilar metal couple from the dropdown list. The database has over six thousand five hundred (6,500) metal-to-metal and metal-to-CFRP composite galvanic couples. The current database includes the following groups of alloys and CFRP composite with over 6,500 galvanic couples:
Aluminum and Its Alloys If you cannot find the galvanic couple of your interest in the list, do let us know through the Contact Us link and we will conduct the necessary tests to generate the required data for inclusion in the software, free of charge for licensed users of GC-Compass (you may have to provide us with the material specimens if they are not readily available).
Figures below show the screenshots of GC-Compass.
Figure 1 GC-Compass Predicts Galvanic Corrosion Rate and Galvanic Compatibility Class for over 6,500 Galvanic Couples.
Figure 2 GC-Compass Predicts Galvanic Corrosion and Galvanic Compatibility Class for over 6,500 Metals, Alloys, and CFRP Composite Galvanic Couples.
After selecting the galvanic couple, the next step is to select the environment relevant to your application. The dropdown list in Figure 3 below has 7 options from seawater to industrial atmosphere, representing the natural environments. If your process fluids are not listed in the dropdown menu, you should choose one of the waters that closely matches the chloride level in your process fluids. WebCorr can customize GC-Compass for your specific process fluids and alloys used in any industry from general engineering to wafer fabrication. A customized version of GC-Compass software can be used to monitor galvanic corrosion of your equipment or facilities in real-time.
Figure 3 GC-Compass Models the Effects of Seven Types of Environments on Galvanic Corrosion and Galvanic Compatibility Class.
The next step is to enter the water velocity and temperature. Water velocity and temperature are critical factors that influence the rate of corrosion. Some galvanic couples such as zinc-steel and aluminum-steel will reverse the polarity at certain temperatures, meaning the usual anodes (zinc and aluminum) have become the cathodes with respect to steel when the temperature exceeds certain threshold value, as shown in Figure 4 below. At temperatures above 60oC, zinc is cathodic to steel, accelerating the corrosion of steel instead of protecting it! Note that it is always the anode that suffers accelerated corrosion due to galvanic coupling. GC-Compass is the only software on the market that models the effect of water velocity and temperature on galvanic corrosion and predicts the polarity reversal when temperature changes.
Figure 4 GC-Compass Predicts the Polarity Reversal for Zinc in Zn-Carbon Steel Couple.
GC-Compass models the effects of temperature and the cathode to anode area ratio on the corrosion rate of the anodic member of the couple and the galvanic compatibility. Other galvanic corrosion prediction software based on the static "curve crossing" methodology simply fail to model the critical effects of water velocity, temperature, and the cathode-to-anode area ratio on the galvanic corrosion rate and galvanic compatibility. The outputs from the GC-Compass software include:
Figure 5 GC-Compass Models the Effects of Temperature and Cathode to Anode Area Ratio on Galvanic Corrosion and Galvanic Compatibility Class.
GC-Compass is not only immensely useful for the prediction of galvanic corrosion and assessment of galvanic compatibility of metals and alloys, it is also equally powerful when used for the prediction of the self-corrosion rate of a single metal when it is not galvanically coupled to another metal.
Service Life Prediction for Fasteners GC-Compass can be a particularly powerful tool for predicting the performance or service life of fasteners. For example, the SS316 fasteners used on AA6061 plates exposed to seawater at 60°C can be accurately predicted using the "effective thickness of anode". Assuming that the fastening assembly will fail when the hole in the AA6061 plates loses 0.35 mm thickness (or the diameter of the hole increases by 0.70 mm), GC-Compass predicts that the SS316-AA6061 assembly exposed to seawater at 60°C would fail in about 6 months (Figure 6a) below.
Figure 6a GC-Compass Predicts the Galvanic Compatibility and the Service Life of Fasteners
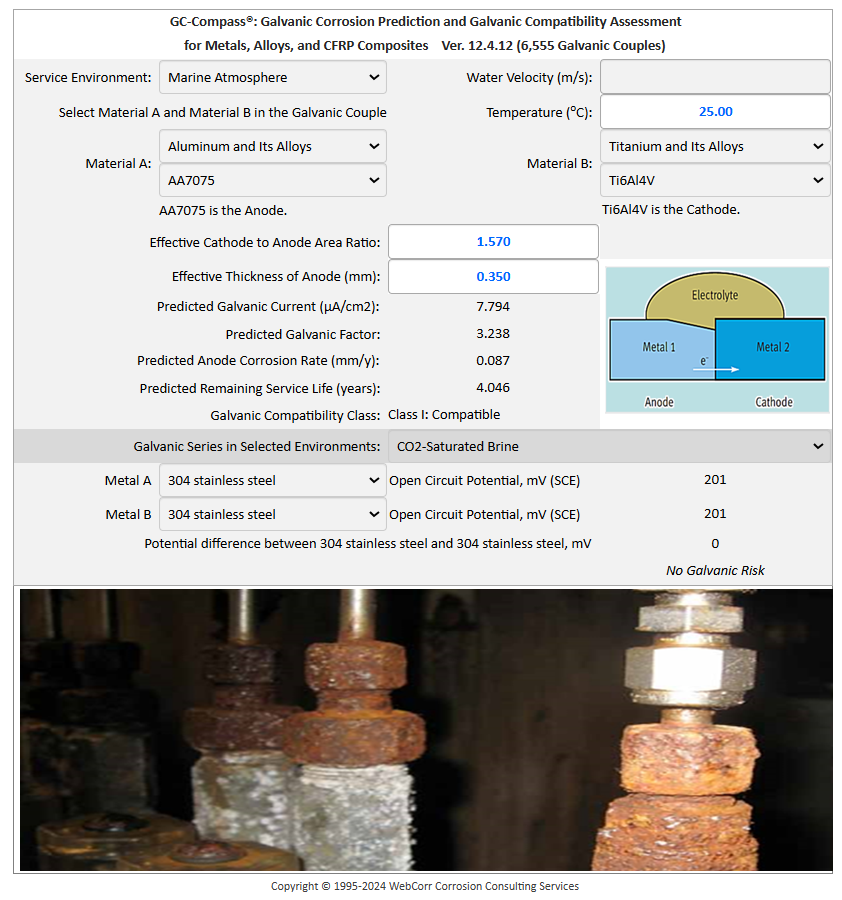
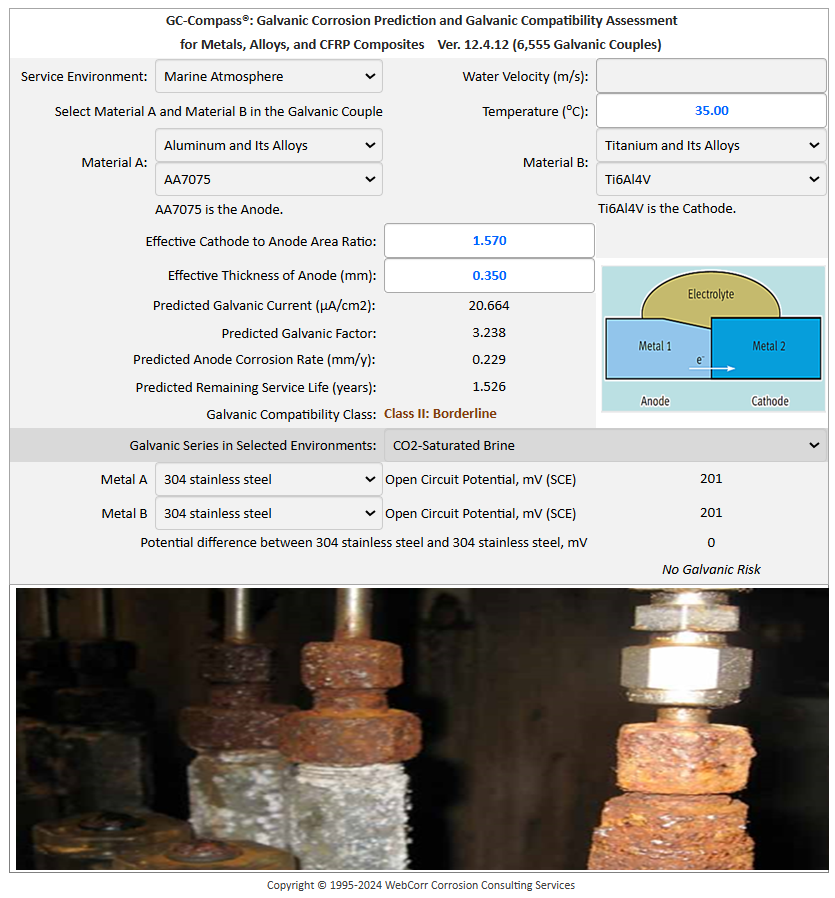
Numerous fastener configurations (metallic materials both ferrous and non-ferrous, with metallic coatings such as cadmium, nickel, zinc and galvanizing, and CFRP composites) exposed to seawater, fresh water, tap water, distilled water, marine atmosphere and industrial atmosphere can be assessed and evaluated at the design stage before service failures occur in the fields. For example, Figure 6b shows that aluminum alloy AA7075 is compatible with titanium alloy Ti-6Al-4V exposed to marine environment at 25°C. No significant galvanic effect is expected under the specified conditions. However, when the temperature is increased by 10°C to 35°C which is entirely expected in aircrafts operating in many parts of the world, the compatibility class shifted to Class II: borderline condition with significant galvanic corrosion rate at 0.229 mm/y, as shown in Figure 6c. Other galvanic corrosion prediction software based on the static "curve crossing" methodology simply fails to model this critical effect of temperature on the galvanic compatibility.
Figure 6b GC Compass Predicts the Compatibility of AA7075 with Ti-6Al-4V Fasteners
Figure 6c GC-Compass Models the Critical Effect of Temperature on the Galvanic Compatibility of Fasteners
Service Life Prediction for Process Piping in Semiconductor Manufacturing In semiconductor manufacturing, the process cooling water is frequently contaminated with copper ions, which will deposit on aluminum alloy AA6061 piping surface and induce pitting corrosion in aluminum AA6061. The temperature of the process cooling water is about 90°C and the pipe wall thickness is 2.85 mm. The first leak in the piping was reported after 850 days in operation. Tap water in GC-Compass closely matches the chloride level in the process cooling water. With this basic information (Figure 7), GC-Compass predicts that the piping would leak in approximately 2.349 years after operation due to the galvanic effect of AA6061 and the copper deposit. Other galvanic corrosion prediction software based on the static "curve crossing" methodology simply fail to model the critical effects of temperature and service environment on the galvanic compatibility and the remaining life.
Figure 7 GC-Compass Predicts Service Life of Process Piping in Semiconductor Manufacturing.
Service Life Prediction for Structures in Marine and Seawater Services AA6061 plate and carbon steel structural members were used for the construction of a vessel for seawater services. The effective cathode to anode area ratio is 14.00. The vessel leaked just over 2 years after commencing operation. The galvanic compatibility between AA6061 and carbon steel predicted by GC-Compass is Class III, which means the two materials are not compatible in the seawater environment, and the time-to-leak predicted by GC-Compass is 2.092 years (Figure 8). Other galvanic corrosion prediction software based on the static "curve crossing" methodology simply fail to model this critical effect of cathode to anode area ratio on the galvanic corrosion rate and galvanic compatibility in seawater.
Figure 8 GC-Compass Predicts the Service Life of Structures in Marine and Seawater Services
Service Life Prediction for Carbon Steel Storage Tank with Stainless Steel 304 Clad Bottom
In a major expansion program, a plant installed several hundred large storage tanks. Most of the older tanks were made of ordinary carbon steel and completely coated on the inside with a baked phenolic paint. The solutions in the tanks were only mildly corrosive to steel but contamination of the product was a major consideration. The coating on the floor was damaged because of mechanical abuse and some maintenance was required. The tops and sides were made of steel, with the sides welded to the stainless steel (304) clad bottoms. The steel was coated with the same phenolic paint, with the coating covering only a small portion of the stainless steel below the weld. A few months after start-up of the new plant, the tanks started failing because of perforation of the side walls. It was observed that most of the holes were located within a 2-inch band above the weld.
Figure 9 GC-Compass Predicts Galvanic Corrosion and the Effect of Cathode-to-Anode Area Ratio in Carbon Steel Storage Tank with SS304 Clad Bottom
Service Life Prediction of a Aluminum Casing of a Water Heater
A hospital needed an emergency power supply in case of mains failure, so a diesel engine was provided. To ensure that the diesel would cut in immediately if the power failed, it was kept warm by means of a water heater with an aluminum casing. After a few months in operation, the 8 mm thick aluminum casing was penetrated causing the water to leak out. Inspection showed that a copper heating element had been used. Copper ions deposited at the 12 o'clock position by convection and caused extremely rapid pitting.
Figure 10 GC-Compass Predicts Pitting Corrosion Due to Galvanic Effect in Aluminium Casing
GC-Compass also predicts galvanic series and the open circuit potentials (free corrosion potentials) for many metals and alloys in selected environments including waters, soils, acids, caustics, oil and gas production wells:
Seawater
As shown in Figure 10 above, the open circuit potential, or the free corrosion potential for mild steel in aerated tap water is predicted by GC-Compass to be -646 mV (SCE) while the potential for copper is 64 mV (SCE). GC-Compass calculates the potential difference between Metal A (mild steel) and Metal B (copper) and concludes that mild steel is anodic to copper in aerated tap water with a very high galvanic corrosion risk.
Figure 11 GC-Compass Predicts Galvanic Series for Metals and Alloys in Selected Environments
Figure 12 GC-Compass Predicts Galvanic Series for Metals and Alloys in Selected Environments
The powerful applications of GC-Compass are truly unlimited in engineering design, galvanic corrosion prediction and modeling, materials compatibility assessment, trouble-shooting process-related issues and failure analysis of components and systems. Figure 14 shows the feature comparison between GC-Compass and other software based on curve-crossing methodology.
Figure 14 GC-Compass Offers More Features Than Other Software Based on Curve-Crossing Methodology.
Click here to contact us for licensing details and experience the power of GC-Compass. |
|
|
GC-Compass, giving you the right directions in Galvanic Corrosion Prediction and Assessment |
|
|
Home | Contact Us | PDF |
Copyright © 1995-2025. All rights reserved. |