|
Source: "Cathodic Protection", by John Morgan, 2nd edition, 1987
Quote: "3.4.6 Groundbed to Structure
Distance.............Suppose a long pipeline is
protected and at the mid-point between groundbeds the anode to pipe distance
is 5 miles while the pipe approaches within 50 yds of the groundbed at its
nearest point. The nearness, or rather the remoteness, of the groundbed may
be estimated by considering the voltage drop that would occur in the
vicinity of the cathode were this structure not present and the groundbed
output was flowing to an infinite cathode. At a point 50 yards from such a
20 amp groundbed in 2,000 ohm cm soil the potential of the ground to
infinity would be
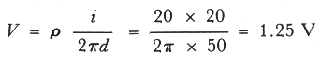
so that a pipeline that was laid to run within 50 yards
of that groundbed could expect a considerable cathodic swing caused by the
positive swing of the earth near the groundbed. If the pipe to groundbed
distance had been 200 yds then the positive swing of the ground relative to
an infinite cathode would be
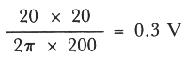
Errors:
In both
equations above, the unit for resistivity "p" is "Ohm.cm"; current "i", amp;
distance "d", "yards". These values are directly plugged into the two
equations without unit conversion.
Corrections:
The
unit for distance "d" must be converted into "cm" before it is plugged into the
equations. The correct calculations are shown below:
For 50 yards, V=(2000 Ohm.cm x 20
Amp)/(2x3.14x4572cm)=1.39 V
For 200 yards, V=(2000 Ohm.cm x 20
Amp)/(2x3.14x18288)=0.35 V
Return to Index
Source: "Corrosion
Prevention by Protective Coatings", 2nd edition, p322
Quote: "Calcareous Deposits Some
coatings seem to be permeable to electrons. In this case, the electrons pass
through the organic coating and are neutralized on the surface of the
coating. When this occurs, the calcareous deposit can buildup on the
exterior of the coating."
Errors:
Electrons can only survive in vacuum, or in metallic or electronic
conductors. Electrons can NOT pass through a non-conductive organic coating.
The formation of calcareous deposit is a chemical reaction that does not
involve the electron-transfer at all.
Corrections:
Discard any of the repeated mention throughout the
book that electrons can pass through the non-conductive organic coatings.
Return to Index
Source:
"Corrosion of Steel in Concrete - Understanding, Investigation and Repair",
by John P Broomfield, 2nd edition, 2007, p236
Quote: "9.5 The Clear/Stratfull
empirical calculation Stratfull developed an empirical
equation to determine the time to first distress of reinforced concrete
in sea water with a know, constant chloride content. Clear modified this to
be used for atmospherically exposed structures.
Errors:
The respective units for "t" and "Z" in Equation (9.4)
are not defined and the equation is wrong.
Corrections:
The correct equation should be:
where the units for d, t and Z are
inches, years and %wt. Return to
Index
|